Today’s world has made clean, safe drinking water a valuable resource. However, different crises, natural catastrophes, and daily living may trigger water shortages. As a prepper, you need to be prepared to handle such problems. With that, can salt water be purified for drinking? Is it considered safe?
Salt water can be purified and made safe for drinking. A procedure known as desalination, which includes distillation, reverse osmosis, and electrodialysis, removes salt and other minerals from the water to make it fit for human consumption.
Read on to learn about the saltwater purification process and techniques for making saltwater drinkable.
Quick Navigation
- Can You Drink Salt Water Alone?
- How To Purify Salt Water For Drinking
- How to Purify Sea Water for Survival In The Wild
- Conclusion
Can You Drink Salt Water Alone?

Saltwater (often called saline water) has significant levels or “concentrations” of dissolved salts. The concentration, in this instance, is the salt content of the water. It’s measured in “parts per million” or ppm.
For example, if the concentration of dissolved salts is 10,000 PPM, 1% of the weight of water is made up of dissolved salts.
What happens if you drink salt water alone?
As part of our everyday diets, we drink liquids, which help to hydrate us and maintain our salt intake at a healthy level. The body’s chemical processes and balances depend on sodium chloride (salt), yet too much sodium may be fatal.
Humans who drink saltwater alone are ingesting too much salt and water into their cells. Although people may consume tiny quantities of salt without harm, saltwater contains significantly more than what the body can metabolize.
When you drink salt water alone, your kidneys will produce more urine; so, you must pee more to eliminate all the extra salt ingested from saltwater.
This cycle creates a vicious effect where the more salt water you drink, the more dehydrated your body becomes, and you eventually die from dehydration.
The Oregon Department of Human Services advises keeping the salt content of drinking water at 250 ppm or less.
While there is no federally mandated limit for salt in drinking water, the US EPA advises keeping it to a maximum of 20 mg/L due to the widespread presence of sodium in other foods and drinks.
Below is the guideline for water’s salt content per US Geological Survey.
| Type | PPM content |
| Safe drinking water/ Freshwater | 250 ppm or less |
| Slightly saline | 1,000 ppm – 3,000 ppm |
| Moderately saline | 3,000 ppm – 10,000 ppm |
| Highly saline | 10,000 ppm – 35,000 ppm |
| Saltwater (Ocean) | About 35,000 ppm |
How To Purify Salt Water For Drinking

Now, how do you make saltwater safe for drinking?
In reality, from the time of the ancient Greeks, humans have been able to make saltwater drinkable.
Umfortunately, purifying salt water on a large scale for cities, states, and countries have traditionally proved to be unaffordable, particularly when compared to using regional and local freshwater resources.
Yet, more communities are converting to seawater to satisfy this essential need as increasing technology pushes prices down, and freshwater becomes more costly and scarce.
Interestingly, many regions where fresh water is scarce are also near the ocean, where desalination is used.
How does desalination work?
Desalination is the process used to remove dissolved salts from water. Nowadays, it is one of the most common methods for obtaining drinkable water for use in agriculture or human consumption when applied to saltwater.
Seawater desalination was mainly utilized aboard ships and submarines during the centuries to provide the crew with fresh water during lengthy voyages.
Nonetheless, this method was not widely accessible until the industrial revolution and, more specifically, until the creation of desalination plants.
Saudi Arabia and the UAE from the Middle East, both desert nations heavily reliant on this method, are the top two countries by volume for desalination plants.
The three primary ways to desalinate water today are the following:
1. Distillation

In the almost 2,400 years since humankind first boiled saltwater and captured the steam in sponges, desalination has advanced significantly. The most popular technique, however, still relies on the same idea: distillation.
In essence, distillation simulates the natural process wherein heated water evaporates into water vapor, leaving behind salts and contaminants. It then condenses as freshwater (aka rain) when it cools to fall.
By using artificial heating, cooling, and water evaporation under decreased air and vapor pressure, distillation facilities streamline and accelerate this process and considerably lower the boiling point of the water used.
Nevertheless, since this process uses a lot of energy, distillation units are often found next to power stations where waste heat may be used to heat the water to a volatile temperature.
2. Reverse Osmosis
Reverse osmosis is another process used to desalinate salty water. In the most basic terms, dissolved salt molecules in the water go through a semipermeable membrane (basically a filter).
Smaller water molecules pass through the membrane pores, but more giant salt molecules do not.
Saline water can be effectively desalinated in a reverse osmosis desalination plant, although it is more costly than other techniques.
Reverse osmosis units should be accessible to desalinate huge volumes of salty water as energy costs decrease in the future. Of the three, reverse osmosis uses less energy for desalination.
3. Electrodialysis
As it genuinely desalts the feed supply, a desalination plant using electrodialysis differs considerably from other practical desalination techniques that extract clean water.
Electrodialysis, or ED, is a pressure-based membrane technology that uses charge-selective membranes.
To put it simply, electrodialysis removes salt and other charged particles from a solution using an electric current. Consider it as a filter that separates the solution from the charged particles in a liquid, such as salt.
A series of membranes with an electric charge separates the charged particles from the solution, producing drinking water or a cleaner, less saline solution.
You may also use this method to clean other liquids, including dissolved salts or other charged particles. Large scale desalination facilities often utilize this method to transform saltwater into potable water.
How to Purify Sea Water for Survival In The Wild
Knowing how to purify seawater into drinking water might spell you between life and death if you ever find yourself alone in the wild without access to fresh drinking water.
Distillation by evaporation is a complex desalination technique used to purify water. In this process of evaporating water, salt and other impurities are removed, making it entirely safe to drink.
Of course, this also implies that to produce condensation; you will also need heat and some kind of trapping.
There are two common ways to purify or distill water in the wild:
Solar Distillation

Solar distillation is the technique of removing salt or other impurities from freshwater using sun energy. Untreated water slowly warms up to high temperatures due to absorbing heat.
The impurities are left behind when the water evaporates, cools, and condenses into vapor due to the heat. Low capacity and independent water-providing systems may employ solar stills.
The process of solar distillation is used in the following survival desalination techniques:
Bottle and can technique: This straightforward desalination technique uses a plastic bottle and a metal can. To make this, cut off the bottle’s bottom, and roll the corners inward to create a gutter at the bottom.

Remove the can’s lid, add saltwater, then cover it with the plastic bottle. Desalinated water will fill the gutter when the evaporation caused by the sun’s heat condenses on the sides of the plastic container and runs down them.
Ensure the bottle’s cap is on, or the moisture or desalinated water will leak into the atmosphere.
Pot and cup method: To use this method, you will need heat-resistant containers and the supplies to make a fire. Put a small bowl or cup in the middle of a big saucepan. Put some saltwater in the saucepan.
Place an upside-down cover on the pot while it warms up. The lid must have a curve or slope for the distilled water to stream toward the center and trickle into the cup, where it gathers for drinking.
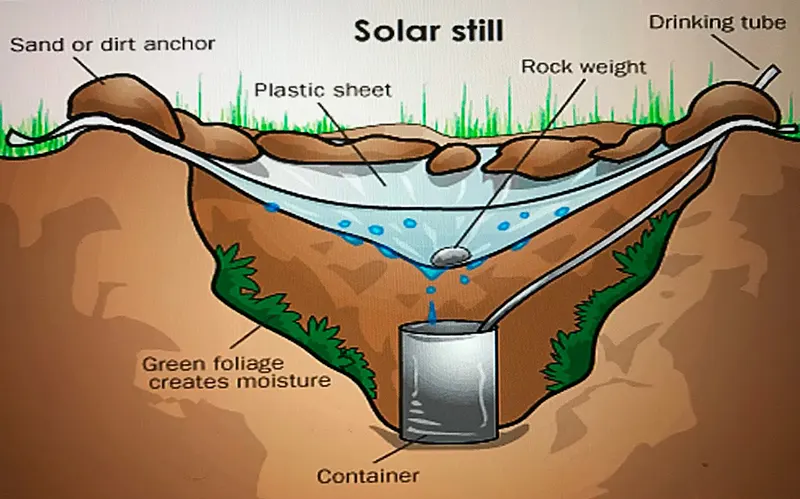
The plastic and hole technique: Place the pot or other container in the center of the hole. Be sure not to fill the hole over the container’s top; pour saltwater into it.
The condensed water will concentrate at a low spot and drop into the container if you stretch transparent plastic over the top of the hole and put a weight—a small rock would do—near the center, right above it. At a location with direct sunshine, this technique works best.
Distillation By Boiling
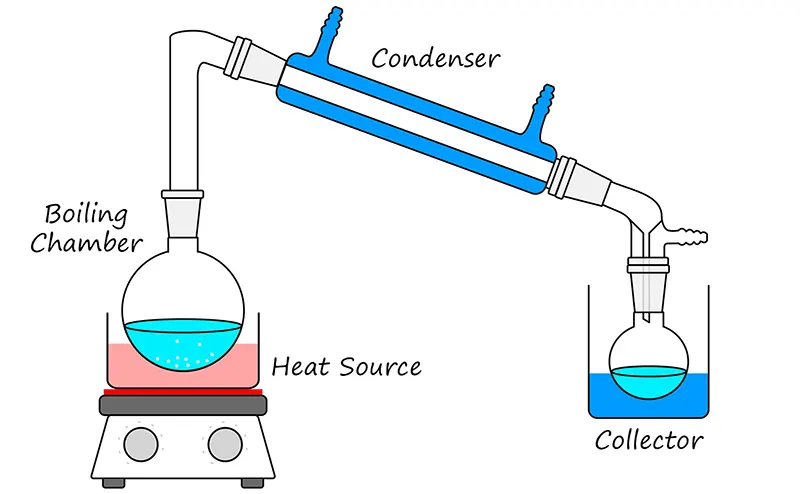
This approach may provide more drinking water than solar distillation, but also needs a lot of heat energy from the outside.
It is comparable to distilling alcohol, which traditionally used a closed container, a condenser, and a third container for collecting the condensed liquid.
The water vapor goes via the condenser or cooling tube, which converts back to the water, which will then be free of salt and other impurities and is then heated to boil the saltwater within the closed container.
Conclusion
In conclusion, you may filter saltwater for drinking using several methods. It’s crucial to remember that these technological desalination techniques may be pricey and need specialist tools, making them unsuitable for emergencies.
Despite this, technological developments are making saltwater purification more and more accessible and inexpensive, providing promise for a more resilient and sustainable future in the face of water scarcity.
To help you become fully prepared for whatever happens, you might want to check out the foods that last forever.
